Abstract
Background:Complete remission rates from induction therapy for Acute Lymphoblastic Leukemia (ALL) in adults have been reported in the range of 80-90%. However, there are many different published regimens and there is no clear consensus for how these patients should be treated. Recent studies have shown that younger adults benefit from pediatric inspired regimens when compared to adult regimens. This has led to the current paradigm where younger patients are treated with pediatric inspired protocols while older patients are treated with any one of a number of chemotherapy regimens. These recommendations are derived from clinical trials data which may not accurately represent outcomes in a real world setting. The purpose of this study was to report on the real world experience of adult ALL patients. The backbone of the CALGB 10102 protocol excluding alemtuzumab has been used at Wake Forest Baptist Hospital for adults with ALL since 2007. This regimen is comprised of an induction cycle termed Module A that consists of dexamethasone, daunorubicin, vincristine, asparaginase, and cyclophosphamide. Upon count recovery this is followed with module B which consists of intrathecal methotrexate, intravenous methotrexate and high-dose cytarabine. Upon count recovery this is followed by module C which consists of repeated doses of IV and IT methotrexate, 3 cycles of each, given every two weeks. Upon completion of module C patients repeat the 3 modules for a total of 6 courses. Patients then begin POMP (6-mercaptopurine [Purinethol], vincristine [Oncovin], methotrexate, and prednisone) maintenance therapy with a goal to complete a total of 2 years of treatment. The goal of this study was to report on the outcomes of adult patients who were treated per this modified CALGB 10102 regimen.
Methods:An IRB-approved retrospective study (June 1, 2007 - December 31, 2015) of 115 adult ALL patients treated at Wake Forest Baptist Hospital with the CALGB 10102 protocol excluding course 4 (alemtuzumab). Philadelphia chromosome positive (Ph+) patients received a tyrosine kinase inhibitor starting on day 15 of module A and continued during all courses and maintenance therapy.
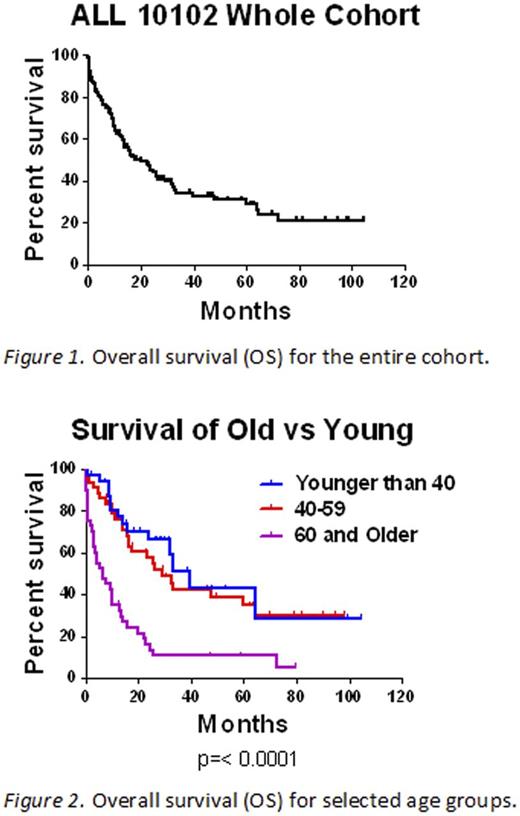
Results: A total of 115 adult patients are evaluable. The median age is 53 (range: 19-86). Sixteen patients were Ph+. Ninety-five patients achieved complete remission (82.6%) and 89 of those (93.6%) received post-remission therapy. Of the 95 patients who achieved remission, 44 patients (46.3%) relapsed. Seven of the 44 relapses (15.9%) involved the CNS. Median survival for the entire cohort was 19.9 months (Figure 1). When stratified by age groups <40, 40-59, and ≥60 years of age, the median overall survival was 39, 29, and 6.6 months respectively (Figure 2). For patients less than 40 years old, 31/32 (96.8%) achieved remission and 17 (54.8%) of those relapsed. For patients 40 to 59 years of age, 39/43 (90.7%) achieved remission and 18 (46.2%) relapsed. In patients 60 years and older, 25/40 (62.5%) achieved remission and 9 (36.0%) of those patients relapsed. From the entire cohort, 12 patients died on or before day 30 (10.4%) and an additional 2 patients died on or before day 60. Of the 14 patients who died on or before day 60, 10 (71.4%) were 60 years of age or older. A univariate analysis revealed that hypertension (0.0008), as well as any comorbidity (0.0003) were significantly associated with failure to achieve complete remission. Of note, the median age of patients with hypertension was 61 years.
Conclusions: Despite strong efforts, the overall survival and relapse rates for adults with Acute Lymphoblastic Leukemia (ALL) remain disappointing. Age continues to be a critical prognostic factor and it should be a consideration in regimen selection. While pediatric inspired protocols have been shown to be superior in young adult patients with ALL, novel regimens are needed for patients 60 years of age and older. We have also demonstrated that in addition to using age as a prognostic factor, concurrent diseases including hypertension may present clinical challenges with regard to achievement of remission.
Ellis: Alexion: Speakers Bureau. Powell: Rafael Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees. Pardee: Rafael Pharmaceuticals: Employment, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal